A study performed by PhD student Vera Wiersma (FGA), from the team of Wiep Scheper, and colleagues reveals that neurons develop granulovacuolar degeneration bodies in response to tau pathology. This collaborative effort was published in Acta Neuropathologica.
Study on granulovacuolar degeneration bodies published in Acta Neuropathologica

GVBs are membrane-enclosed structures containing a vacuolar space with a dense core. They are found in the brains of patients with various neurodegenerative disorders, including Alzheimer’s disease. These disorders are collectively called tauopathies, since the intracellular aggregation of the protein tau is a key neuropathological feature. Up till now, how and why GVBs arise in tauopathy patient brains was unclear.
The present study, with FGA PhD student Vera Wiersma as first author, shows that tau pathology is the cause of GVB formation. Based on this finding, novel models to study GVBs in cells and mice are presented. These models led the FGA team and their Amsterdam UMC and international collaborators to conclude that GVBs are lysosomal structures.
A method called “tau seeding” was used to induce intracellular tau pathology in neurons in vivo and in vitro. Subsequently, GVBs were found in neurons with tau pathology. GVBs did not develop in cultured glia cells, indicating that GVB formation is neuron-selective response to tau pathology. Using automated microscopy, a strong positive correlation was found between the extent of tau pathology and the number neurons with GVBs: more tau pathology, more GVBs!
Applying confocal, EM and STED super-resolution microscopy in primary mouse neurons, the authors show that the GVB membrane is a single lipid bilayer immunopositive for lysosomal membrane markers. Lysosomes are organelles that degrade waste delivered to them from either inside or outside the cell. In line with this, GVBs contained markers of degradative activity. Therefore, GVBs may actively contribute to cellular waste disposal. Interestingly, the dense GVB core contains selectively targeted proteins.
In conclusion, this work sheds light on the formation and identity GVBs: a long overlooked neuropathological hallmark of tauopathies. Future studies will determine the functional implications of GVB formation: is this response to tau pathology beneficial or detrimental for a neuron?
For the full article, published open access, see: https://rdcu.be/bPJry
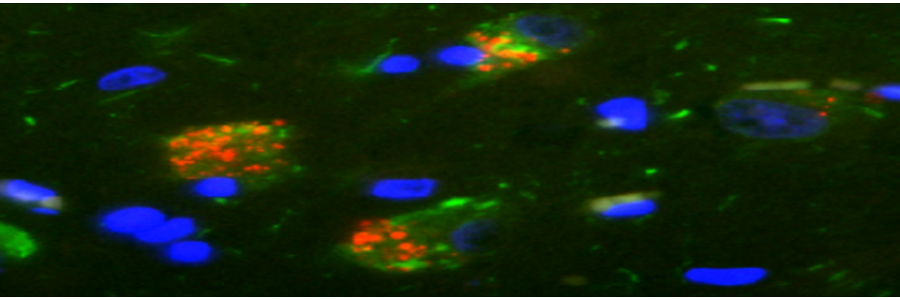
GVBs are shown in red, tau pathology in green, a neuron-specific marker in gray and cell nuclei in blue. GVBs can be observed in cultured primary mouse neurons with seeded tau pathology (condition: PFF), but never in control buffer-treated cultures (condition: Ctrl).
